PRODUCT IDENTIFICATION
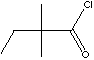
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
clear to yellow liquid
REFRACTIVE INDEX
GENERAL DESCRIPTION & APPLICATIONS
Pivalic acid is the shortest chain tert-carboxylic acid. Atoms within a molecule occupy space. When atoms are crowded together and overlapped electron clouds, van der Waals repulsions produce an steric hindrance. Steric hindrance may influence conformational equilibria and reactivity. Although steric hindrance is sometimes a unfavorable structure due to less readily reaction, it can provide an escape from undesired side-reactions, can affect varying degrees of rate and energy and can produce the target derivatives which are more resistant to hydrolysis and oxidation than the derivatives from linear chain. Pivalic acid is a key intermediate for the target molecules which require hydrolytic stability and a variety of chemical resistance. Pivalic acid is used mainly in the form of chloride salt (pivaloyl chloride) which are obtained commercially from phosgene. It is used as an intermediate in the production of peroxides and peroxy-esters required for the polymer and agrochemical production. It is used as an intermediate to prepare pharmaceuticals ( Ampicillin, Amoxycillin, Cephalosporins). 2,2-Dimethylbutyric acid is the next shortest chain tert-carboxylic acid which has similar applications with pivalic acid.
APPEARANCE
clear to yellowish liquid
99.0% min
0.5% max